A crystal consists of a space array of atoms or molecules built up by regular repetition in three dimensions of some fundamental structural unit. The electronic energy level for single free atom do not apply to the same atom in a crystal.
When atoms form crystals, it is found that the energy levels of the inner-shell electrons are not affected by the presence of the neighboring atoms. However the levels of the outer-shell electrons are changed and these electrons are shared by more than one atom in the crystal.
A brief discussion of the energy band structure of one element which consisting of N atoms shows below.
Fig.2
Imagine that it is possible to vary the spacing between atoms without altering the type of fundamental crystal structure. If the atoms are so far apart that the interaction between them is negligible, the energy levels will coincide with those of the isolated atoms.
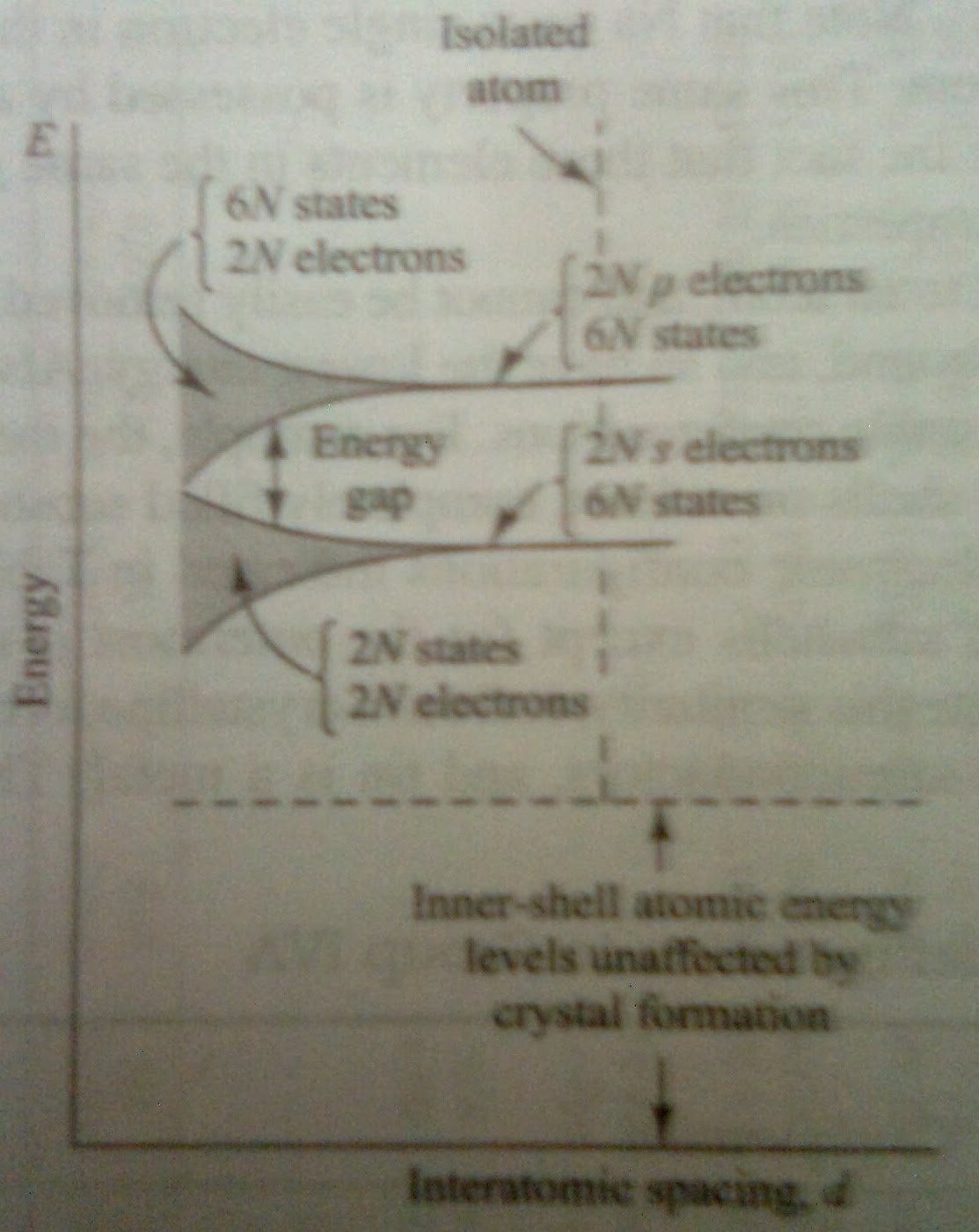
For the taken element the outer two subshells contains 2 s electrons and 2 p electrons. Indicated to the extreme right in fig.1a, there are 2N electrons completely filling the 2N possible s levels. all at the same energy level. Since the p atomic subshells has 6 possible states which fill only 1/3rd of the 6N possible p states, all at the same energy level.
Fig.2a
If we now decrease the interatomic spacing of our imaginary crystal(moving right to left in Fig.2a), an atom will exert an electric force on its neighbors.
Hence, the 2N degenerate s states must spread out in energy. The separation between levels is small, but since N is very large, the total spread betweenthe minimum and maximum energy may be several electrons volts if the interatomic distance is decreased sufficiently. This large number of discrete but closely spaced energy levels is called an "Energy Band" and indicated by the shaded region in Fig.2a.
In Fig.2b, small enough distances these bands will overlap. Under such conditions the 6N upper states merge with the 2N lower states, giving a total of 8N levels, half of which are occupied by the 2N + 2N = 4N available electrons.
At this spacing each atom has given up four electrons to the band, these electrons can no longer be said to orbit in s or p subshells of an isolated atom, but rather they belong to the crystal as a whole. In this sense taken element is tetravalent, since they contribute four electrons each to the crystal. The band these electrons occupy is called the "Valence Band".
If the spacing between atoms is decreased below the distance at which the bands overlap, the interaction between atoms is indeed large. At the crystal-lattice spacing, we find the valence band filled with 4N electrons separated by a forbidden band of extent Eg from an empty band consisting of 4N additional states. This upper band is called the "Conduction Band".
God Bless Thx.......